Submucosal tumors (SMT) of the gastrointestinal tract are elevated lesions originating from the muscularis mucosa, submucosa, or muscularis propria, and may also be extraluminal lesions. With the development of medical technology, traditional surgical treatment options have gradually entered the era of minimally invasive treatment, such as laparoscopic surgery and robotic surgery. However, in clinical practice, it can be found that "surgery" is not suitable for all patients. In recent years, the value of endoscopic treatment has gradually received attention. The latest version of the Chinese expert consensus on endoscopic diagnosis and treatment of SMT has been released. This article will briefly learn the relevant knowledge.
1.SMT epidemic characteristics
(1) The incidence of SMT is uneven in various parts of the digestive tract, and the stomach is the most common site for SMT.
The incidence of various parts of the digestive tract is uneven, with the upper digestive tract being more common. Of these, 2/3 occur in the stomach, followed by the esophagus, duodenum, and colon.
(2)The histopathological types of SMT are complex, but most SMT are benign lesions, and only a few are malignant.
A.SMT includes non-neoplastic lesions such as ectopic pancreatic tissue and neoplastic lesions.
B.Among the neoplastic lesions, gastrointestinal leiomyomas, lipomas, Brucella adenomas, granulosa cell tumors, schwannomas, and glomus tumors are mostly benign, and less than 15% can appear as tissue Learn evil.
C.Gastrointestinal stromal tumors (GIST) and neuroendocrine tumors (NET) in SMT are tumors with certain malignant potential, but this depends on its size, location and type.
D.The location of SMT is relatedto the pathological classification: a. Leiomyomas are a common pathological type of SMT in the esophagus, accounting for 60% to 80% of esophageal SMTs, and are more likely to occur in the middle and lower segments of the esophagus; b.The pathological types of gastric SMT are relatively complex, with GIST, leiomyoma and ectopic pancreas being the most common. Among gastric SMT, GIST is most commonly found in the fundus and body of the stomach, leiomyoma is usually located in the cardia and upper part of the body, and ectopic pancreas and ectopic pancreas are most common. Lipomas are more common in the gastric antrum; c. Lipomas and cysts are more common in the descending and bulbous parts of the duodenum; d. In SMT of the lower gastrointestinal tract, lipomas are predominant in the colon, while NETs are predominant in the rectum.
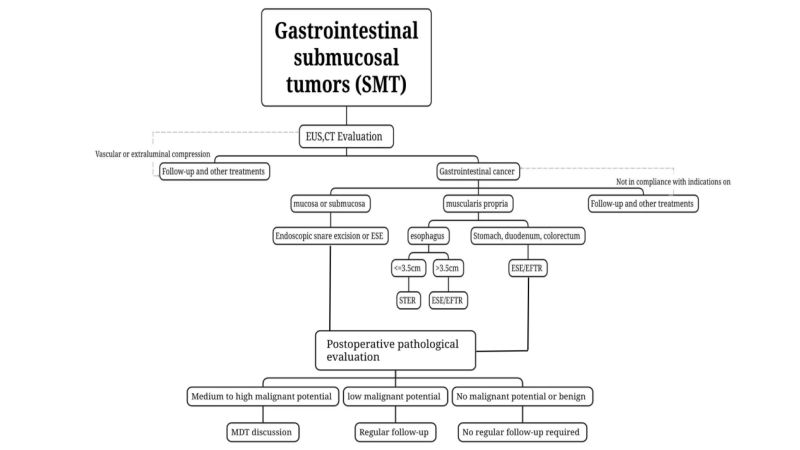
(3)Use CT and MRI to grade, treat, and evaluate tumors. For SMTs that are suspected of being potentially malignant or have large tumors (longdiameter > 2 cm), CT and MRI are recommended.
Other imaging methods, including CT and MRI, are also of great significance for the diagnosis of SMT. They can directly display the location of tumor occurrence, growth pattern, lesion size, shape, presence or absence of lobulation, density, homogeneity, degree of enhancement, and boundary contour, etc., and can find whether and the degree of thickening of the gastrointestinal wall.More importantly, these imaging examinations can detect whether there is invasion of adjacent structures of the lesion and whether there is metastasis in the surrounding peritoneum, lymph nodes and other organs. They are the main method for clinical grading, treatment and prognosis assessment of tumors.
(4)Tissue sampling is not recommended for benign SMTs that can be diagnosed by conventional endoscopy combined with EUS, such as lipomas, cysts, and ectopic pancreas.
For lesions suspected of being malignant or when conventional endoscopy combined with EUS cannot assess the benign or malignant lesions, EUS-guided fine-needle aspiration/biopsy can be used(endoscopic ultrasonography guided fine needle aspiration/biopsy, EUS-FNA/FNB), mucosal incision biopsy(mucosalincision-assisted biopsy, MIAB), etc. perform biopsy sampling for preoperative pathological evaluation. In view of the limitations of EUS-FNA and the subsequent impact on endoscopic resection, for those who are eligible for endoscopic surgery, on the premise of ensuring that the tumor can be completely resected, units with mature endoscopic treatment technology can be treated by experienced The endoscopist performs endoscopic resection directly without obtaining preoperative pathological diagnosis.
Any method of obtaining pathological specimens before surgery is invasive and will damage the mucosa or cause adhesion to submucosal tissue, thereby increasing the difficulty of surgery and possibly increasing the risks of bleeding, perforation, and tumor dissemination. Therefore, preoperative biopsy is not necessarily necessary. Necessary, especially for SMTs that can be diagnosed by conventional endoscopy combined with EUS, such as lipomas, cysts, and ectopic pancreas, no tissue sampling is required.
2.SMT endoscopic treatment
(1)Treatment principles
Lesions that have no lymph node metastasis or very low risk of lymph node metastasis, can be completely resected using endoscopic techniques, and have a low risk of residual and recurrence are suitable for endoscopic resection if treatment is necessary. Complete removal of the tumor minimizes residual tumor and the risk of recurrence. Theprinciple of tumor-free treatment should be followed during endoscopic resection, and the integrity of the tumor capsule should be ensured during the resection.
(2)Indications
i.Tumors with malignant potential suspected by preoperative examination or confirmed by biopsy pathology, especially those suspected of GIST with a preoperative assessment of a tumor length of ≤2cm and a low risk of recurrence and metastasis, and with the possibility of complete resection, can be endoscopically resected; for tumors with a long diameter For suspected low-risk GIST >2cm, if lymph node or distant metastasis has been excluded from preoperative evaluation, on the premise of ensuring that the tumor can be completely resected, endoscopic surgery may be performed by experienced endoscopists in a unit with mature endoscopic treatment technology. resection.
ii. Symptomatic (eg, bleeding, obstruction) SMT.
iii.Patients whose tumors are suspected to be benign by preoperative examination or confirmed by pathology, but cannot be followed up regularly or whose tumors enlarge within a short period of time during the follow-up period and who have a strong desire for endoscopic treatment.
(3)Contraindications
i. Identify the lesions that have metastasized to lymph nodes or distant sites.
ii. For some SMT with clear lymphnodeor distant metastasis, bulk biopsy is required to obtain pathology, which can be regarded as a relative contraindication.
iii. After detailed preoperativeevaluation, it is determined that the general condition is poor and endoscopic surgery is not possible.
Benign lesions such as lipoma and ectopic pancreas generally do not cause symptoms such as pain, bleeding, and obstruction. When SMT manifests as erosion, ulcer, or rapidly increases in a short period of time, the possibility of it being a malignant lesion increases.
(4)Choice of resection method
Endoscopic snare resection: ForSMT that is relatively superficial, protrudes into the cavity as determined by preoperative EUS and CT examinations, and can be completely resected at one time with a snare, endoscopic snare resection can be used.
Domestic and foreign studies have confirmed that it is safe and effective in superficial SMT <2cm, with a bleeding risk of 4% to 13% and a perforationrisk of 2% to 70%.
Endoscopic submucosal excavation,ESE : For SMTs with a long diameter ≥2 cm or if preoperative imaging examinations such as EUS and CT confirm that the tumor protrudes into the cavity, ESE is feasible for endoscopic sleeve resection of critical SMTs.
ESE follows the technical habits ofendoscopic submucosal dissection (ESD) and endoscopic mucosal resection, and routinely uses a circular “flip-top” incision around the tumor to remove the mucosa covering the SMT and fully expose the tumor. , to achieve the purpose of preserving the integrity of the tumor, improving the radicalness of surgery, and reducing intraoperative complications. For tumors ≤1.5 cm, a complete resection rate of 100% can be achieved.
Submucosal Tunneling Endoscopic Resection, STER : For SMT originating from the muscularis propria in the esophagus, hilum, lesser curvature of the gastric body, gastric antrum and rectum, which are easy to establish tunnels, and the transverse diameter is ≤ 3.5 cm, STER can be the preferred treatment method.
STER is a new technology developed based on peroral endoscopic esophageal sphincterotomy (POEM) and is an extension of ESD technology. The en bloc resection rate of STER for SMT treatment reaches 84.9% to 97.59%.
Endoscopic Full-thickness Resection,EFTR :It can be used for SMT where it is difficult to establish a tunnel or where the maximum transverse diameter of the tumor is ≥3.5 cm and is not suitable for STER. If the tumor protrudes under the purple membrane or grows outside part of the cavity, and the tumor is found to be tightly adherent to the serosa layer during surgery and cannot be separated, it can be used. EFTR performs endoscopic treatment.
Proper suturing of the perforationsite after EFTR is the key to the success of EFTR. In order to accurately assess the risk of tumor recurrence and reduce the risk of tumor dissemination, it is not recommended to cut and remove the resected tumor specimen during EFTR. If it is necessary to remove the tumor in pieces, the perforation needs to be repaired first to reduce the risk of tumor seeding and spread. Some suturing methods include: metal clip suture, suction-clip suture, omental patch suture technique, "purse bag suture" method of nylon rope combined with metal clip, rake metal clip closure system (over the scope clip, OTSC) OverStitch suture and other new technologies to repairs gastrointestinal injuries and dealing with bleeding, etc.
(5)Postoperative complications
Intraoperative bleeding: Bleeding that causes the patient's hemoglobin to drop by more than 20 g/L.
To prevent massive intraoperative bleeding,sufficient submucosal injection should be performed during the operation to expose larger blood vessels and facilitate electrocoagulation to stop bleeding. Intraoperative bleeding can be treated with various incision knives, hemostatic forceps or metal clips, and preventive hemostasis of exposed blood vessels found during the dissection process.
Postoperative bleeding: Postoperative bleeding manifests as vomiting blood, melena, or blood in the stool. In severe cases, hemorrhagic shock may occur. It mostly occurs within 1 week after surgery, but can also occur 2 to 4 weeks after surgery.
Postoperative bleeding is often related tofactors such as poor postoperative blood pressure control and corrosion of residual blood vessels by gastric acid. In addition, postoperative bleeding is also related to the location of the disease, and is more common in the gastric antrum and low rectum.
Delayed perforation: Usually manifests as abdominal distension, worsening abdominal pain, signs of peritonitis, fever, and imaging examination shows gas accumulation or increased gas accumulation compared with before.
It is mostly related to factors such as poor suturing of wounds, excessive electrocoagulation, getting up too early to move around, eating too earl, poor blood sugar control, and erosion of wounds by gastric acid. a. If the wound is large or deep or the wound has fissure-like changes, the bed rest time and fasting time should be appropriately extended and gastrointestinal decompression should be performed after surgery (patients after lower gastrointestinal tract surgery should have anal canal drainage); b. Diabetic patients should strictly control their blood sugar; those with small perforations and mild thoracic and abdominal infections should be given treatments such as fasting, anti-infection, and acid suppression; c. For those with effusion, closed chest drainage and abdominal puncture can be performed Tubes should be placed to maintain smooth drainage; d. If the infection cannot be localized after conservative treatment or is combined with severe thoracoabdominal infection, surgical laparoscopy should be performed as soon as possible, and perforation repair and abdominal drainage should be performed.
Gas-related complications: Including subcutaneous emphysema, pneumomediastinum, pneumothorax and pneumoperitoneum.
Intraoperative subcutaneous emphysema (shown as emphysema on the face, neck, chest wall, and scrotum) and mediastinal pneumophysema (swelling of the epiglottis can be found during gastroscopy) usually do not require special treatment, and the emphysema will generally resolve on its own.
Severe pneumothorax occurs during surgery [airway pressure exceeds 20 mmHg during surgery
(1mmHg=0.133kPa), SpO2<90%, confirmed by emergency bedside chest X-ray], surgery can often be continued after closed chest drainage.
For patients with obvious pneumoperitoneum during the operation, use a pneumoperitoneum needle to puncture the McFarland pointin the right lower abdomen to deflate the air, and leave the puncture needle in place until the end of the operation, and then remove it after confirming that no obvious gas is discharged.
Gastrointestinal fistula: Digestive fluid caused by endoscopic surgery flows into the chest or abdominal cavity through a leak.
Esophageal mediastinal fistulas and esophagothoracic fistulas are common. Once a fistula occurs, perform closed chest drainage to maintain smooth drainage and provide adequate nutritional support. If necessary, metal clips and various closing devices can be used, or the full covering can be recycled. Stents and other methods are used to block thefistula. Severe cases require prompt surgical intervention.
3.Postoperative management (follow-up)
(1) Benign lesions: Pathology suggests that benign lesions such as lipoma and leiomyoma do not require mandatory regular follow-up.
(2) SMT without malignant potential: For example, rectal NETs 2cm, and medium- and high-risk GIST, complete staging should be performed and additional treatments (surgery, chemoradiotherapy, targeted therapy) should be strongly considered. treat). The formulation of the plan should be based on multidisciplinary consultation and on an individual basis.
(3) Low malignant potential SMT: For example, low-risk GIST needs to be evaluated by EUS or imaging every 6 to 12 months after treatment, and then treated according to clinical instructions.
(4) SMT with medium and high malignant potential: If postoperative pathology confirms type 3 gastric NET, colorectal NET with a length >2cm, and medium- and high-risk GIST, complete staging should be performed and additional treatments (surgery, chemoradiotherapy, targeted therapy) should be strongly considered. treat). The formulation of the plan should be based on [about us 0118.docx]multidisciplinary consultation and on an individual basis.
We, Jiangxi Zhuoruihua Medical Instrument Co.,Ltd., is a manufacturer in China specializing in the endoscopic consumables, such as biopsy forceps, hemoclip, polyp snare, sclerotherapy needle, spray catheter, cytology brushes, guidewire, stone retrieval basket, nasal biliary drainage catheter etc. which are widely used in EMR, ESD, ERCP. Our products are CE certified, and our plants are ISO certified. Our goods have been exported to Europe, North America, Middle East and part of Asia, and widely obtains the customer of the recognition and praise!
Post time: Jan-18-2024


