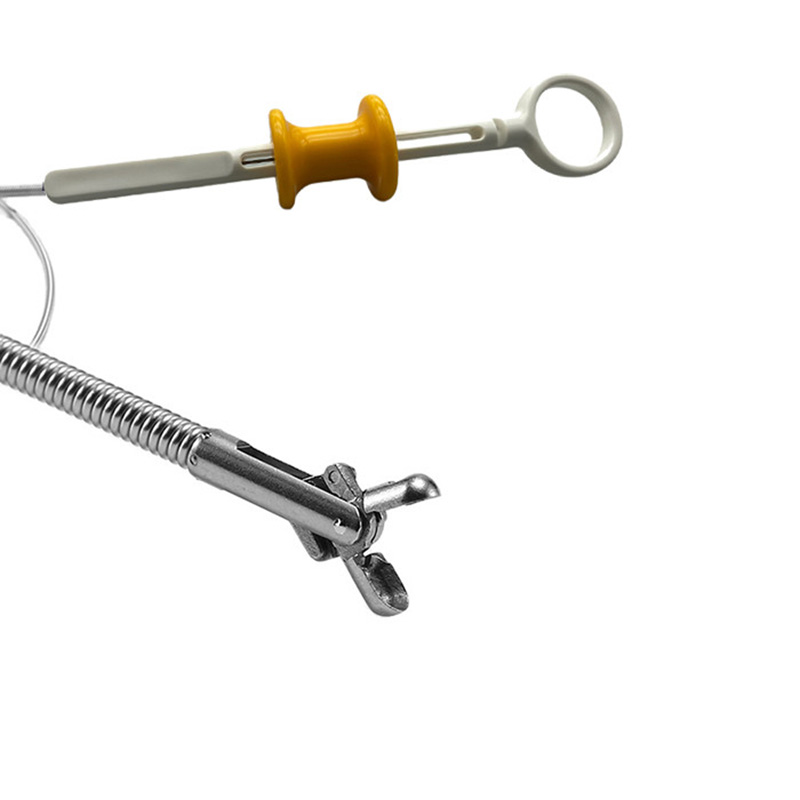
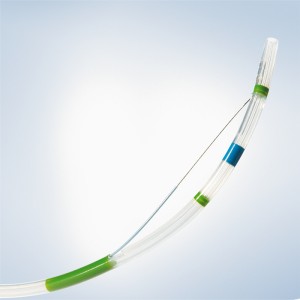
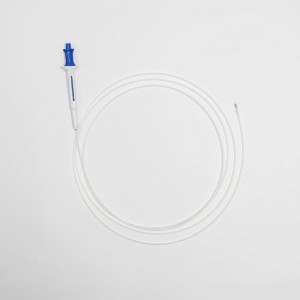
Disposable Endoscopic Uterine Urology Ureteral Biopsy Forceps for Medical Use
Disposable Endoscopic Uterine Urology Ureteral Biopsy Forceps for Medical Use
Application
Urology forceps available for use during Flexible Cystoscopy
Specification
| Model | O.D. Φ(mm) | Working Length L(mm) | Jaw Type | Characters |
| ZRH-BFA-1506-PWL | 1.55 | 600 | Oval | Non-coated, without spike |
Our Market

Our products are not only sold in China, but also exported to Europe, South and East Asia, the Middle East and other oversea market.
FAQs
Q: CAN I REQUEST AN OFFICIAL QUOTATION FROM YOU ON THE PRODUCTS?
A: Yes, you can contact us to request a free quote, and we will respond within the same day .
Q: WHAT ARE YOUR OFFICIAL OPENING HOURS?
A: Monday to Friday 08:30 - 17:30. Weekends Closed.
Q: IF I HAVE AN EMERGENCY OUTSIDE THESE TIMES WHOM CAN I CALL?
A: In all emergencies please call 0086 13007225239 and your enquiry will be dealt with as soon as possible.
Q: WHY SHOULD I BUY FROM YOU?
A: Well why not? - We provide quality products, professional friendly service, with sensible pricing structures; Working with us to save money, but NOT at the expense of Quality.
Q: COULD YOU PROVIDE FREE SAMPLES?
A: Yes,free samples or trial order are available.
Q: WHAT IS THE AVERAGE LEAD TIME?
A: For samples, the lead time is about 7 days. For mass production, the lead time is 20-30 days after receiving the deposit payment. The lead times become effective when (1) we have received your deposit, and (2) we have your final approval for your products. If our lead times do not work with your deadline, please go over your requirements with your sale. In all cases we will try to accommodate your needs. In most cases we are able to do so.
Q: DO YOUR PRODUCTS CONFORM TO INTERNATIONAL STANDARDS?
A: Yes, The suppliers that we work with all conform to International Standards of manufacturing such as ISO13485, and conform to Medical Device Directives 93/42 EEC and are all CE compliant.