On January 13, the sixth batch of national medical consumables centralized procurement (hereinafter referred to as "national procurement of medical consumables") was bid in Tianjin.
At 7:30, bidding companies began entering the venue to submit their application materials.
At 9:30, the submission of application materials by companies ended; a total of 496 products from 227 companies submitted bids.
At 11:30, the first round of bid announcements concluded; according to the rules, companies that were not selected in the first round have the opportunity to submit bids in a second round, encouraging more companies to supply at appropriate prices and enriching clinical options.
Continuing the rules of the fifth batch of medical consumables national procurement, this bidding round still offers two quotation opportunities. However, what's different is that this round has introduced a landmark "anchor price" mechanism. This mechanism replaces the previous "N-fold minimum bid" approach in national medical consumables procurement. It uses the average range of on-site quotations from shortlisted enterprises as the evaluation benchmark, eliminates pre-set threshold bid prices, and replaces administrative pricing with dynamic game theory.
Starting from the 11th batch of national drug procurement at the end of 2025, the principles of "stabilizing clinical practice, ensuring quality, preventing circumvention of bidding, and countering involution" have been incorporated into the national procurement guidelines, shifting the overall direction from "competition" to "stability."
In this centralized procurement optimization, we have clearly established rules against "involution". Instead of simply selecting the lowest price to calculate price differentials, when the lowest price is excessively low, we use 65% of the average shortlisted price as the benchmark for price differential control. Among the 20 competitive groups, this rule was triggered in 8 groups, playing a crucial role in preventing individual companies' excessively low bids from dragging down the overall product prices in the same group.
According to on-site information, 440 products from 202 enterprises were finally selected. The selection rate of enterprises in this centralized procurement reached 89%, and the product selection rate also exceeded 89%.
From the results, it appears that foreign enterprises have collectively "withdrawn" from the bidding for Urological Consumables.
The National Medical Insurance Administration stated that the selected results are expected to be implemented around May 2026, at which time patients across the country will have access to high-quality and affordable products selected through centralized procurement.
*The above and following data are manually statistics for reference only, and the official version shall prevail.
Urological Interventional Foreign Enterprises Withdraw Collectively, While Domestic Companies Achieve High Bid-Winning Rates
This urological intervention category includes 8 product categories such as ureteral intervention guidewires and intervention sheaths, with a total demand exceeding 25 million units. Ureteral intervention guidewires have the highest demand (1,372,386 units).
l Urological intervention consumables are used in stone removal surgery for patients with kidney stones and ureteral stones. Different surgical plans require different types of consumables, involving complex products, which were previously a "blank area" in centralized procurement.
A total of 454 products from 195 companies participated in the bidding for urological intervention consumables, and 398 products from 170 companies were selected. The selection rate for companies is approximately 87%, and the selection rate for products is approximately 88%.
Furthermore, manufacturers of drug-eluting scored balloons and pressure-measuring soft lens catheters with special functions have all been selected, which can effectively meet the needs of clinical special scenarios.
From the specific selection results,
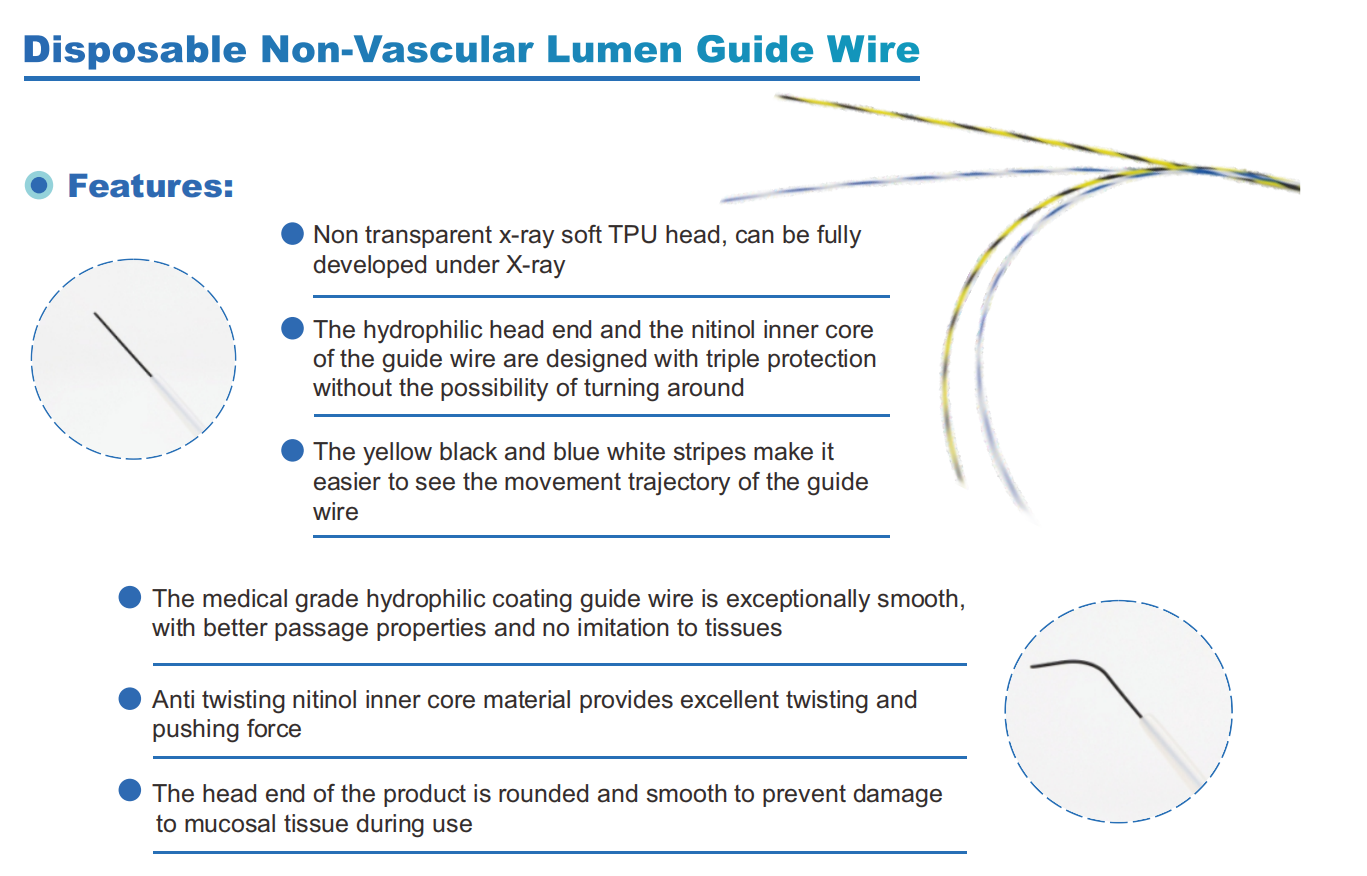
Ureteral Guidewires were selected from 92 enterprises, with a selection rate of approximately 77%. The list includes:
l Reborn Medical, Copper, Laikai medical, Innovex Medical, Wellead, ZRHmed etc. Group A shortlisted candidates
l Cook, Bard, and Boston Scientific from foreign enterprises did not get selected.
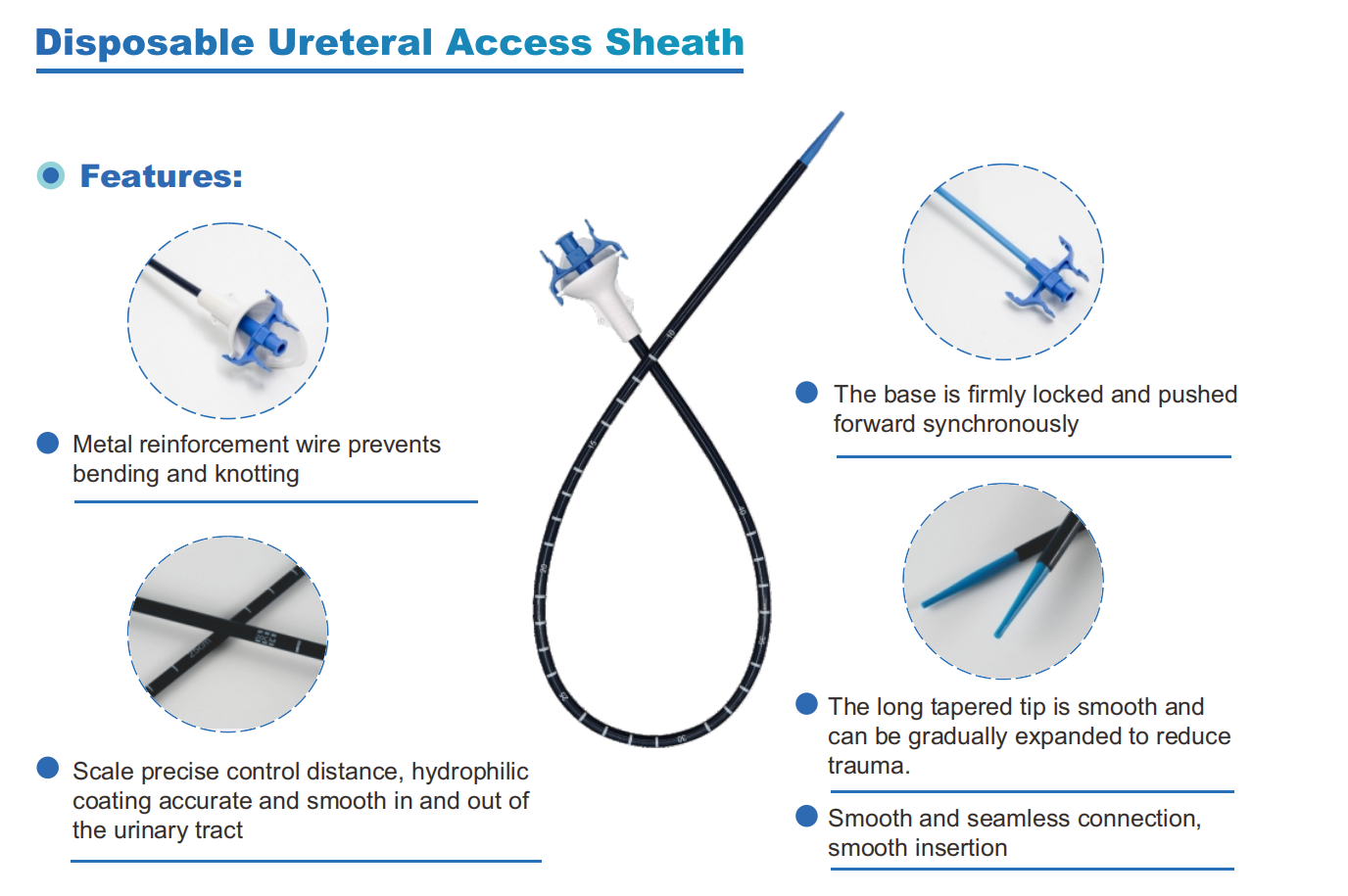
Ureteral Access Sheath (without physiological pressure measurement function at the target site), products from 84 enterprises were shortlisted for selection, with a selection rate of approximately 78.5%. The list includes:
l Reborn Medical, Suzhou Huamei, Copper, MicroPort® Urocare, YIGAO, Innovex Medical, Wellead Medical, ZRHmed etc. Group A shortlisted candidates
l Cook, Bard from foreign enterprises did not get selected.
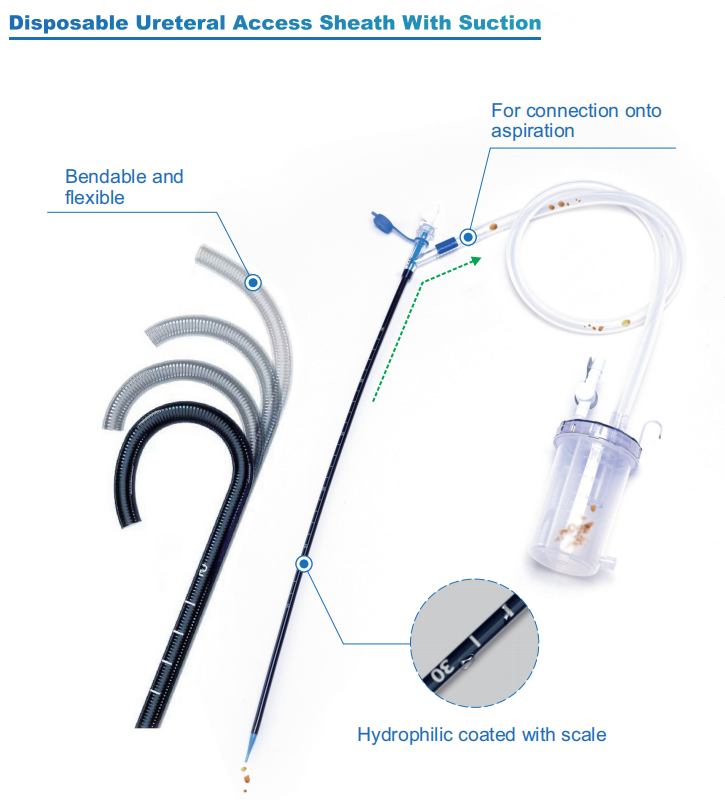
For Ureteral Access Sheath with Suction (equipped with the function of measuring physiological pressure at the target site), three companies' products were shortlisted, achieving a 100% success rate. These companies are: YIGAO, Inventor Technology, and ZRHmed and ZSR Biomedical Technology, all of whom were shortlisted.
Ureteral balloon dilation catheters: 31 companies' products were shortlisted for selection, with a selection rate of approximately 94%. These include:
l Innovex Medical, Wellead Medical, Bard (a foreign company), and YIGAO Group A are the proposed winners;
l Cook (foreign company) is expected to be selected in Group B; Boston Scientific was not selected.
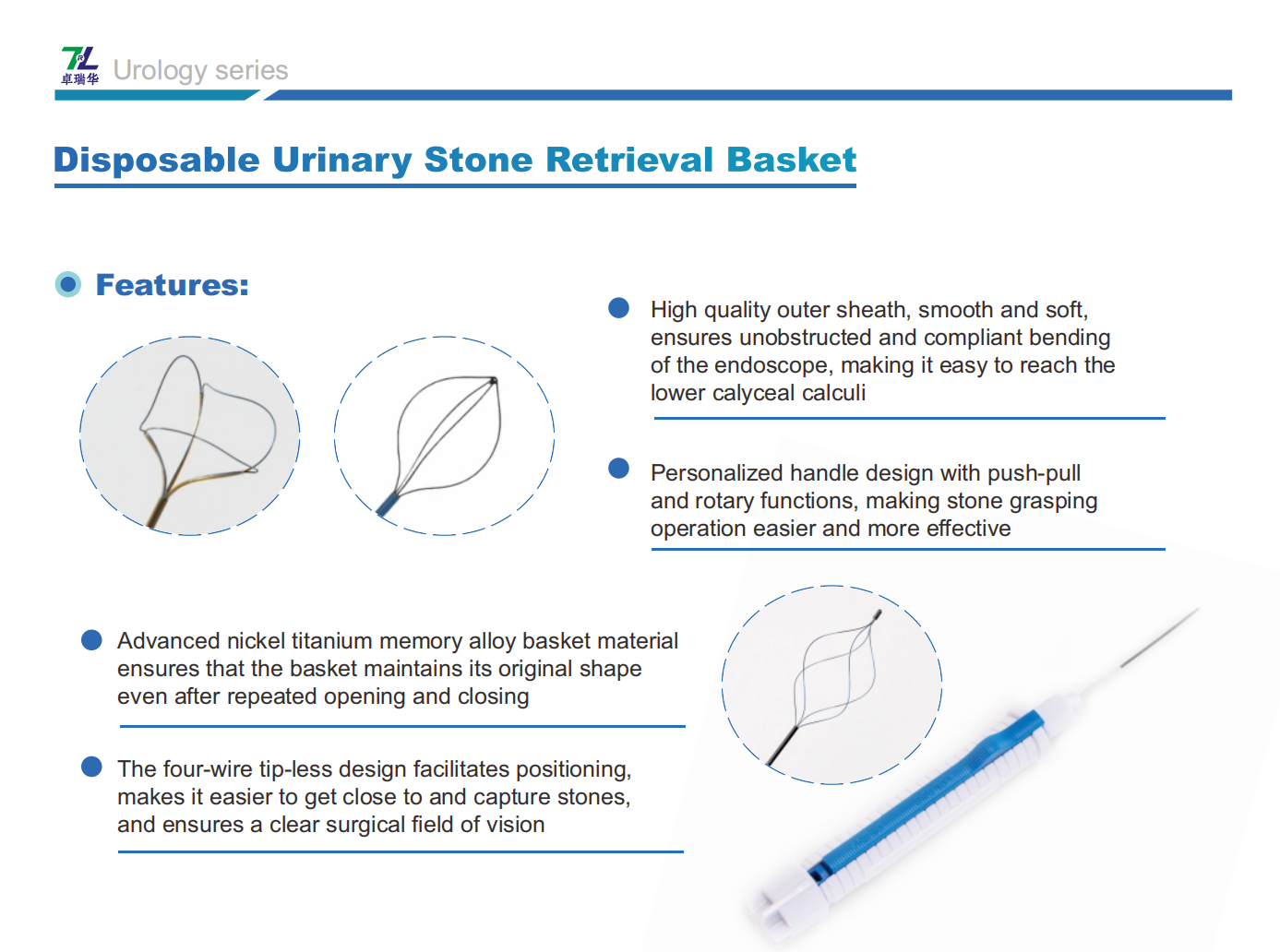
For Urinary Stone Retrieval Baskets, 63 companies' products were shortlisted, with a selection rate of approximately 75%. These include:
l Reborn Medical, Innovex Medical, Wellead Medical, ZRHmed, Copper, and Boston Scientific (a foreign company) are among the companies nominated for Group A.
l Foreign companies such as Cook (who reported the highest volume) and Bard were not selected.
Disposable flexible ureteroscope catheters (without the function of measuring physiological pressure at the target site) were shortlisted for selection, with 73 companies' products selected, representing a selection rate of approximately 77%. These include:
l PUSEN, Happiness Works Medical, REDPINE, ,Shanghai An Qing Medical, Reborn Medical and other companies are expected to be selected in Group A;
l Foreign companies such as KARL STORZ were not selected.
For disposable flexible ureteroscope catheters (equipped with the function of measuring physiological pressure at the target site), all products from 4 companies were shortlisted for selection, achieving a 100% success rate. These companies are: Happiness Works Medical, Plug and Play, Creek Medical (0 submissions), and YIGAO (0 submissions).
For nephrostomy kits, 42 companies' products were shortlisted, representing a selection rate of approximately 56%. These include:
l Reborn Medical, Laikai medical, Wellead Medical, Copper, YIGAO, Innovex Medical, and others are among the companies nominated for Group A.
l Foreign companies such as CREATE MEDIC (ranked among the top three in terms of volume) and Cook were not selected.
After the implementation of this centralized procurement, the market share of foreign enterprises' products is expected to be further compressed.
Meanwhile, to ensure the supply quality of products after procurement, the Medical Insurance Bureau has made efforts.
On one hand, to prevent low prices from compromising clinical safety, this round of rules emphasizes full intervention by drug regulatory authorities and implements "two full coverages":
full coverage inspection - conducting surprise inspections at production sites of all selected enterprises;
full coverage sampling - focusing on random testing of batches of low-price selected products.
Once non-compliance with supply contracts or substandard quality is detected: the winning bid qualification will be cancelled, the enterprise will be listed on the violation list; and the enterprise will be suspended from participating in national centralized procurement for a certain period in the future.
On the other hand, today's national procurement is no longer a "closed list system" but a "rolling admission system". That is, during the procurement cycle, newly approved products or unreported varieties of selected enterprises can be directly listed online under the premise of accepting the selected price.
We, Jiangxi Zhuoruihua Medical Instrument Co.,Ltd., is a manufacturer in China specializing in the endoscopic consumables, include GI line such as biopsy forceps, hemoclip, polyp snare, sclerotherapy needle, spray catheter, cytology brushes, guidewire, stone retrieval basket, nasal biliary drainage catheter etc. which are widely used in EMR, ESD, ERCP. And Urology Line, such as ureteral access sheath with suction, ureteral access sheath, disposable Urinary Stone Retrieval Basket, and urology guidewire etc.
Our products are CE certified, and our plants are ISO certified. Our goods have been exported to Europe, North America, Middle East and part of Asia, and widely obtains the customer of the recognition and praise!
Post time: Jan-15-2026