In recent years, an emerging force that cannot be ignored is rising – domestic endoscope brands. These brands have been making breakthroughs in technological innovation, product quality, and market share, gradually breaking the monopoly of foreign companies and becoming the “domestic star” in the industry.
24 in total, listed in no particular order.
Shanghai Aohua Endoscopy Co., Ltd., established in 1994, is headquartered at No.66, Lane 133, Guangzhong Road, Minhang District, Shanghai. As a high-tech enterprise specializing in the research, production, and sales of electronic endoscopy equipment and endoscopic surgical consumables, it was listed on the STAR Market on November 15, 2021 (stock code: 688212). The company’s products include electronic upper gastrointestinal endoscopes, electronic bronchoscopes, etc., which are applied in clinical departments such as gastroenterology, respiratory medicine, and otolaryngology. In 2023, the company achieved operating revenue of 678 million yuan.
In 2005, the company launched its independently developed electronic endoscopy system VME-2000; in 2013, it released the AQ-100 system with spectral staining function; and in 2016, it entered the field of endoscopic consumables through the acquisition of Hangzhou Jingrui. In 2018, it launched the optical-electronic endoscopy system AQ-200, and in 2022, it released its first 4K ultra-high definition endoscopy system AQ-300. In 2017, it was recognized as a high-tech enterprise.
Shenzhen SonoScape Bio-Medical Electronics Co., Ltd. (Stock Code: 300633) is a globally leading technology company committed to independent research and manufacturing of medical devices. The company product portfolio covers ultrasound medical imaging, endoscopic diagnosis and treatment, minimally invasive surgery, and cardiovascular intervention. The company provide specialized integrated solutions for medical institutions in over 170 countries and regions worldwide. SonoScape aspires to become a technological force protecting global health, creating more possibilities for life.
The company emphasize technological innovation and have established overseas R&D centers since our inception. To date, the company has set up seven major R&D centers in San Francisco and Seattle (USA), Tuttlingen (Germany), Tokyo (Japan), as well as Shenzhen, Shanghai, and Wuhan (China). By integrating global leading technology resources and continuous R&D investment, the company maintain our core technological advantages. SonoScape is dedicated to providing more accurate and efficient medical solutions through technological innovation, working together with medical professionals to deliver superior diagnostic and treatment services for patients worldwide.
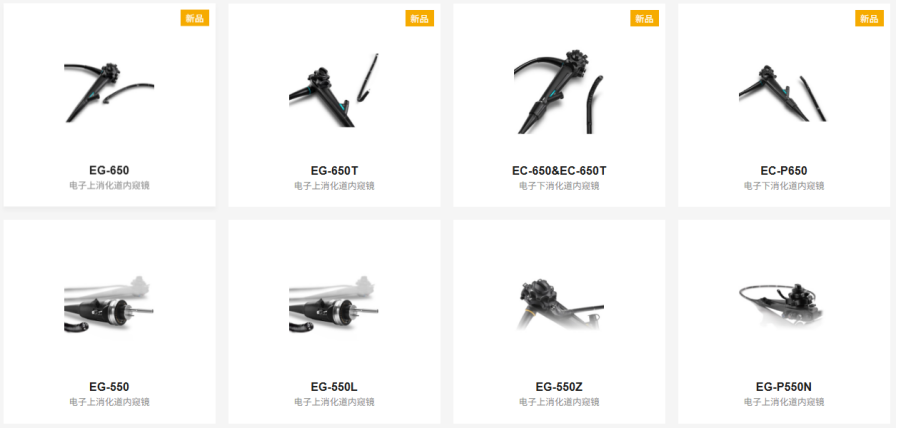
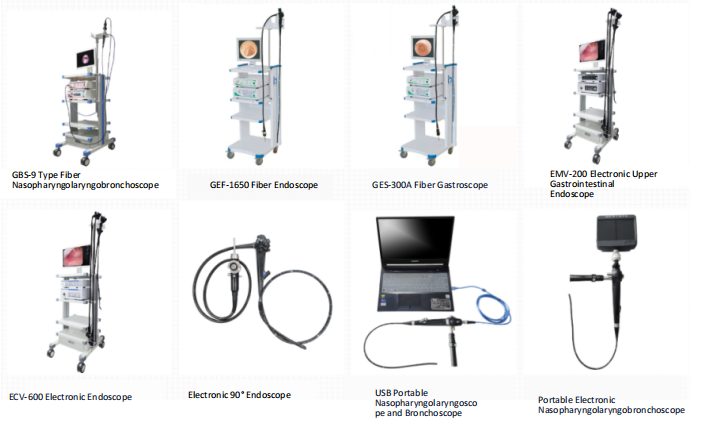
Shanghai Endo View Medical Equipment Co., Ltd., located in Caohejing Hi-Tech Economic Development Zone, Shanghai, is an integrated enterprise specializing in research, development, production, sales, and service. It combines high-tech elements of medical endoscopy optics, mechanics, and electronics. As China’s first company to introduce advanced foreign fiber bundle technology and apply it to product markets, we specialize in manufacturing various medical endoscopes, endoscopic cold light sources, and related peripheral equipment, as well as providing surgical instrument maintenance services.
The company is a member unit of the Shanghai Medical Equipment Industry Association. Our products strictly comply with the national medical device product registration and licensing system. We have registered with the State Administration for Industry and Commerce and obtained exclusive rights to the “Endoview” and “Outai” product trademarks. Endo View holds the “Medical Device Production Enterprise License (No. 20020825 issued by Shanghai Drug Administration, License Class: Class III Medical Products)” and the “People’s Republic of China Medical Device Operating Enterprise License”. Endo View has also obtained the CE certificate issued by TUV. The company vigorously implements the quality policy of “Establishing Quality Fundamentals and Creating the Outai Brand” to achieve our corporate culture philosophy of creating value for customers. Endo View has passed ISO9001 and ISO13485 quality system certifications, covering products including fiber bronchoscopes, fiber choledochoscopes, fiber nasopharyngolaryngoscopes, electronic gastroscopes, electronic enteroscopes, and medical cold light sources.
Founded in October 2016, Scivita Medical is a minimally invasive medical device company specializing in the research, development and commercialization of medical endoscopes and related innovative products.
With a vision of “Rooted in China, Looking at the World”, the company’s headquarters and R&D base are located in Suzhou Industrial Park, while subsidiaries and branches have been established in Tokyo, Shanghai, Chengdu, Nanjing and other cities.
Relying on its strong independent research capabilities and unique core technology platform, Scivita Medical develops innovative and high-quality endoscopic minimally invasive diagnosis and treatment solutions including “reusable endoscopes + disposable endoscopes + accessories”, covering multiple clinical departments such as general surgery, gynecology, hepatobiliary surgery, urology and respiratory intervention. The products have been sold to multiple countries and regions worldwide.
Adhering to the corporate values of “Focus on Clinical Needs”, “Collaborative Innovation”, “People-Oriented” and “Excellence and Efficiency”, Scivita Medical will continuously upgrade its core minimally invasive diagnosis and treatment technologies, improve market penetration through superior product capabilities, and become the preferred brand trusted by doctors and patients worldwide.
Guangdong OptoMedic Technology Co., Ltd. was established in July 2013, with its headquarters located in Foshan, Guangdong. It has set up marketing centers in Beijing and Shanghai, as well as product research and development and industrialization centers in Suzhou, Changsha, and Shangrao. OptoMed focuses on the research and production of high-end medical devices, including full-featured endoscopic imaging platforms, fluorescent laparoscopes, white light laparoscopes, electronic flexible endoscopes, disposable endoscopes, fluorescent imaging agents, and energy device consumables.
As a national-level “Little Giant” enterprise specializing in niche markets, OptoMedic has four national and provincial innovation platforms. It has received three national key research and development project approvals during the “13th Five-Year Plan” and “14th Five-Year Plan” periods, won two China Patent Awards, one first prize and one second prize for provincial scientific and technological progress. Meanwhile, OptoMedic has been awarded titles such as National High-tech Enterprise, National Intellectual Property Advantage Enterprise, Guangdong Intellectual Property Demonstration Enterprise, and Guangdong Manufacturing Single Champion Enterprise. It also has a Guangdong New Research and Development Institution and a Guangdong Engineering Technology Research Center. OptoMedic is one of the earliest domestic enterprises to obtain NMPA registration certificates and has acquired multiple international certifications.
Established in 1937, the company originated as the Medical Equipment Workshop of Shanghai New Asia Sanitary Materials Co., Ltd., which was later renamed as Shanghai Medical Optical Instrument Factory. After several restructuring reforms, it was officially established as Shanghai Medical Optical Instrument Co., Ltd. in 2008. Our products cover most fields of medical flexible endoscopes, making us a professional domestic endoscope research, development and manufacturing enterprise. As renowned Chinese endoscope brands, both “SMOIF” and “Shanghai Medical Optical” have continuously improved our R&D technological capabilities. Historically, we successfully developed China’s first optical fiber image bundle and the first medical optical fiber gastroscope with electric bulb illumination, winning numerous national and Shanghai Science and Technology Progress Awards. The company and its products have been honored with titles such as “Shanghai High-tech Enterprise,” “Shanghai Medical Equipment Quality Product,” “Shanghai Medical Equipment Industry 5-star Integrity Enterprise,” and “Shanghai Medical Equipment Manufacturer Quality Credit Grade Enterprise.”
The company has always striven to promote the “precision and reliability” quality policy, having passed ISO9001 and ISO13485 quality system certifications. Our products have gained widespread market trust, establishing a solid presence in the domestic market while also exporting to international markets.
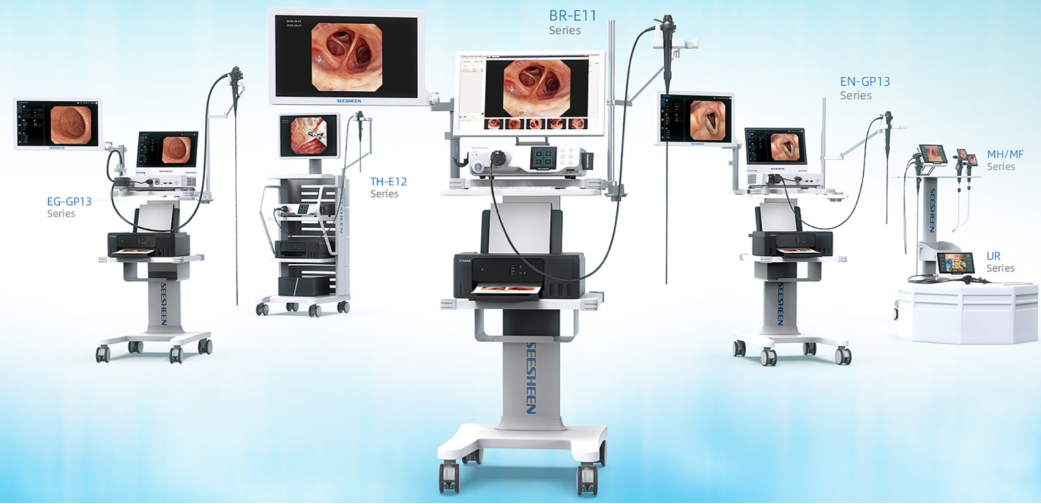
SEESHEEN, established in 2014, is a national high-tech enterprise and a national-level “Little Giant” specialized in the research, development, production, and sales of medical endoscope products, as well as providing technical services. The company’s main products include medical flexible endoscopes, covering reusable endoscopes, disposable endoscopes, and animal endoscopes. Meanwhile, we provide customers with endoscope clinical training, product maintenance, and after-sales services.
Seizing the opportunity of endoscope localization, the company embarked on a path of independent research and development. Through continuous technological breakthroughs and product optimization, it has successfully developed a product matrix that rivals imported products in stability and precision while offering affordable prices. The company now holds over 160 authorized national patents and has established a comprehensive layout including reusable endoscopes, disposable endoscopes, and veterinary endoscopes. With excellent performance and superior quality, its products have been sold to more than 3,000 medical institutions worldwide.
In the future, the company will continue to adhere to the strategy of “innovation-driven development and product service for clinical needs”. We will always practice our corporate values of “customer first, employee-oriented, team cooperation, and innovative progress”. We aim to fulfill our mission of “making medical endoscopy diagnosis and treatment technology more accessible to the public” and achieve our vision of becoming a “world-renowned medical endoscope manufacturer”.
Shenzhen INNERMED is a technology-based small and medium-sized enterprise (2024), high-tech enterprise (2024), and small micro enterprise. The company was established on May 26, 2015 and is located at Room 601, Building D, Block 1, Phase 1 of Chuangzhi Yuncheng, Liuxian Avenue, Xili Community, Xili Street, Nanshan District, Shenzhen. Currently in operation, its business scope includes: research, development and sales of Class I medical supplies and equipment, electronic products, and mechanical equipment; domestic trade (excluding exclusively operated, controlled, and monopolized commodities); import and export business (except for projects prohibited by laws, administrative regulations, and State Council decisions, restricted projects must obtain permission before operation); investment in industrial projects (specific projects to be reported separately); production and operation of Class II and III medical devices; etc. The company’s brand projects include Yingmeida.
Founded in 2010, Zhejiang UE MEDICAL focuses on visual, precise, intelligent, and remote diagnosis and treatment of respiratory and digestive systems. As a national high-tech enterprise, UE MEDICAL is a pioneer in domestic airway management, a global endoscopy technology innovator, and a provider of visual medical system solutions, integrating R&D, manufacturing, sales, and service.
UE MEDICAL has always adhered to the concept of “from clinical practice to clinical application”. We have established collaborations with multiple universities, research institutions, and hospital experts. UE MEDICAL has a Zhejiang Provincial Enterprise Technology Center and Research Institute. UE MEDICAL has hold over 100 patents in fields such as visual airway management, endoscopy, telemedicine, artificial intelligence, and mixed reality. Our core products have passed FDA registration in the United States, CE certification in the European Union, and KFDA certification in South Korea. UE MEDICAL has been awarded titles such as “Specialized, Refined, Pioneering and Innovative Small Giant Enterprise by the Ministry of Industry and Information Technology” and “Zhejiang Province Hidden Champion Enterprise”.
Guangdong Insighters Medical Technology Co., Ltd., established in 2020, is a wholly-owned subsidiary of Shenzhen Insight Medical Technology Co., Ltd., located in Meizhou High-tech Industrial Park. The company focuses on the research, development, production and sales of innovative visualization medical devices. Insighters products are widely used in clinical disciplines such as anesthesia, respiratory, critical care, ENT, and emergency departments. The users span nearly 100 countries worldwide, including the United States and European Union, making them one of the innovative leaders in the global visualization airway management field. The company emphasizes research and development innovation as well as quality management, holding dozens of patents in visualization airway management, endoscopy, and telemedicine. Insighters has a self-built 45,000 square meter high-standard factory, including nearly 10,000 square meters of Class 10,000 and Class 100,000 clean production workshops. Insighters has independent laboratories for complete physical and chemical, microbiological testing, a full active medical device production line, and sterilization facilities. Insighters can undertake contracted research, development, and production of active and sterile medical devices.
Shenzhen HugeMed was established in 2014, headquartered in Shenzhen, the city of innovation. As a medical device enterprise committed to providing cutting-edge endoscopic diagnosis and treatment solutions worldwide, it has been awarded dual certifications as a National High-tech Enterprise and a “Little Giant” Specialized, Refined, Pioneering and Innovative Enterprise. With a professional team of over 400 people covering the entire chain of R&D, production, sales and service, the company occupies an office and production space exceeding 20,000+ square meters.
To become a key force in promoting endoscopic diagnosis and treatment for the general public, Shenzhen HugeMed has stayed true to its people-oriented mission, focusing on independent research and development and global strategic. The company has mastered multiple core technologies and accumulated over 100 invention patents, launching disposable and reusable endoscopic products covering various medical fields including anesthesiology, respiratory medicine, ICU, urology, general surgery, gastroenterology, and gynecology. Our products have obtained multiple international certifications including NMPA, CE, FDA, and MDSAP, selling well in over 100 countries and regions both domestically and globally. HugeMed has successfully installed and applied our products in more than 10,000 medical institutions worldwide, continuously providing efficient and reliable medical support for global patients and healthcare professionals.
MINDSION is not an impetuous and rash enterprise; it is more like a scholar who prefers quiet contemplation. MINDSION understands the importance of expertise and regards research and development as the fundamental principle of its existence. As early as 1998, its founder, Mr. Li Tianbao, dedicated himself to the medical industry and has since been focused on scientific research of new-generation medical technologies. In 2008, he began in-depth development in the field of endoscopy. After 25 years of technological accumulation and dedicated research spanning more than a generation, we have successfully expanded into a brand-new and highly promising field of portable electronic endoscopy. By pioneering truly original Chinese technology, MINDSION has become “another eye for doctors,” and we are fortunate to have achieved “excellence in technology.”
MINDSION is not an enterprise seeking quick success and instant benefits; it is more like a traveler crossing thousands of mountains. MINDSION firmly believes in the power of continuous innovation, working tirelessly day and night to overcome various technical challenges, creating three world-firsts – the world’s first wireless electronic endoscope, the world’s first portable endoscope, and the world’s first ergonomic fingerprint-molded endoscope. The intelligence and miniaturization of its high-definition wireless endoscopes have reached a level very close to the world’s most advanced technology. MINDSION’s perfect domestic has brought leapfrog development to the field. Focusing on the blue ocean market, the research and development of disposable endoscopes have pushed MINDSION to the forefront of major trends, and we are eager to create another “source of value.”
Since its establishment in 2001, Shanghai HUGER has been a professional developer and manufacturer of medical endoscopy systems. It has two R&D centers in Shanghai and Beijing, and two manufacturing factories in Shanghai and Zhejiang. HUGER is committed to developing endoscopy systems with optimal performance, featuring excellent image quality, high operability, and reliable quality. Meanwhile, HUGER has a professional after-sales service team to provide customers with timely, effective, and satisfactory service, as well as professional training in system maintenance. HUGER’s products are sold in more than 70 countries and regions worldwide. HUGER is seeking partners to join hands and move forward together!
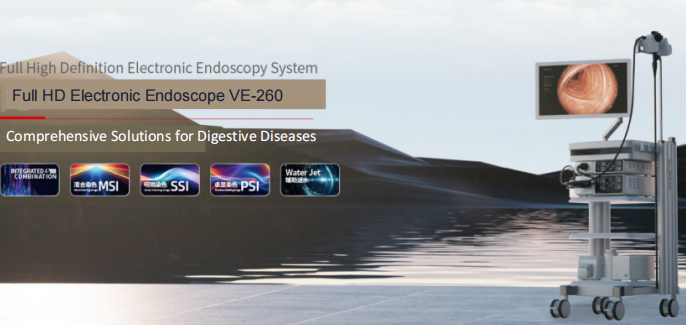
Over the years, Chongqing Jinshan Technology Group Co., Ltd. has focused on independent research, development, production, and service of high-end minimally invasive medical product technologies, providing comprehensive intelligent diagnosis and treatment solutions for digestive diseases. Today, Jinshan has grown into a national-level “Little Giant” enterprise specializing in digital medical equipment research, development, production, sales, and service, serving as the leading unit for “Artificial Intelligence Medical Device Innovation Tasks” by the Ministry of Industry and Information Technology and the National Medical Products Administration. Jinshan holds a pivotal position in the global digestive healthcare field.
With microsystem MEMS technology as its core, Jinshan has undertaken dozens of national-level research programs including the National “863 Program,” National Science and Technology Research Program, and International Cooperation Program. Jinshan has successfully developed dozens of medical devices at the international leading level, including capsule endoscopes, capsule robots, full HD electronic endoscopy systems, electronic gastrointestinal endoscopes, digestive tract pressure detection systems, and pH capsules. Currently, the company’s patent portfolio has exceeded 1,300 patents.
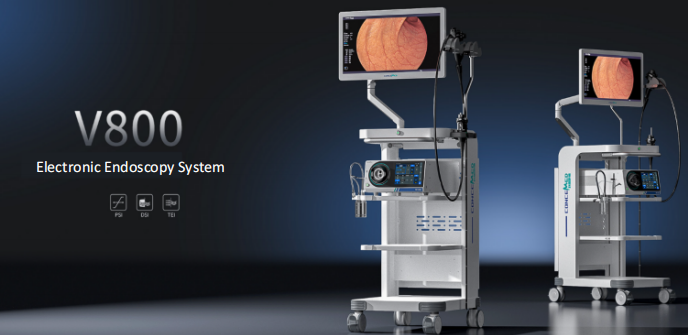
Established in 2022 by a visionary and passionate founding team, CONCEMED has assembled talents from leading international and domestic medical technology companies and top universities, fully participating in and driving the development, iteration, and breakthroughs of domestic endoscopy.
Since its inception, CONCEMED has gained recognition and support from global leading venture capital firms and industrial capital. It has secured continuous investments from venture capital institutions including Legend Capital, National Innovation Center for High-performance Medical Devices (NIC), and IDG Capital, obtaining essential funding, experience, and resources for long-term development, which provides a solid foundation for the company’s future growth.
Hangzhou LYNMOU Medical Technology Co., Ltd. (hereinafter referred to as LYNMOU ) was established in Hangzhou in 2021, and simultaneously set up a Shenzhen R&D center and a Hangzhou manufacturing base. The founding team consists of experienced domestic and international senior engineers and experts with many years (an average of 10 years) of medical device industry experience. The team has gathered talents from leading medical technology companies and top universities both domestically and internationally. The core team has led and driven the technological development, commercialization, and globalization process of domestic endoscopes from scratch. The company’s product expertise covers computer tissue optical imaging, hardware technology, flexibleware technology, high-precision mechanical design, materials science, and process design. It has innovatively proposed the concept of “full-scenario imaging,” with various special light imaging modes fully covering the imaging needs of different clinical scenarios, providing professional imaging solutions for the entire screening, diagnosis, and treatment process of early gastrointestinal cancer.
Relying on its solid R&D capabilities and extensive manufacturing experience, LYNMOU quickly obtained product approvals. The company’s first domestically developed full-scene imaging electronic endoscopy system VC-1600 series, as well as electronic upper and lower gastrointestinal endoscopes, were officially approved in April-May 2024. While obtaining product certifications, LYNMOU also completed a tens of millions of RMB Pre-A financing round. In July, the company completed the installation of the first set of equipment, and gradually established marketing and after-sales service systems, successfully achieving the commercial landing from R&D to marketing. Moving forward, LYNMOU will continue to expand its market presence, benefiting doctors and patients with high-quality products and services, while empowering the healthcare industry.
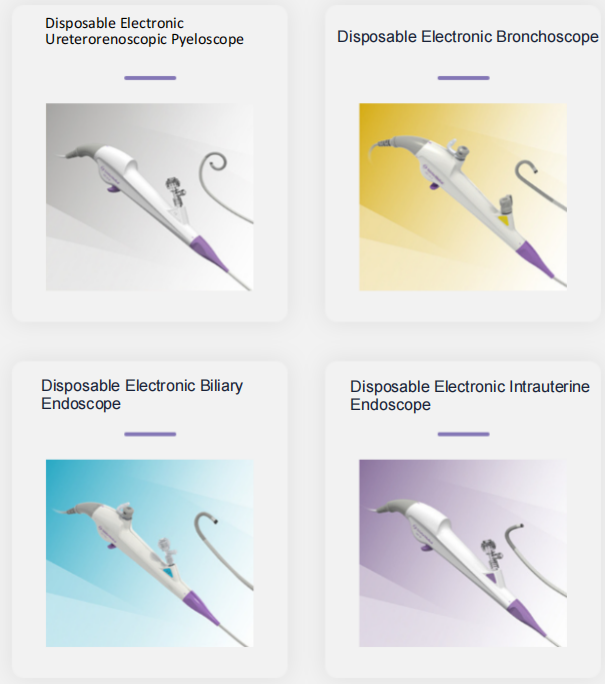
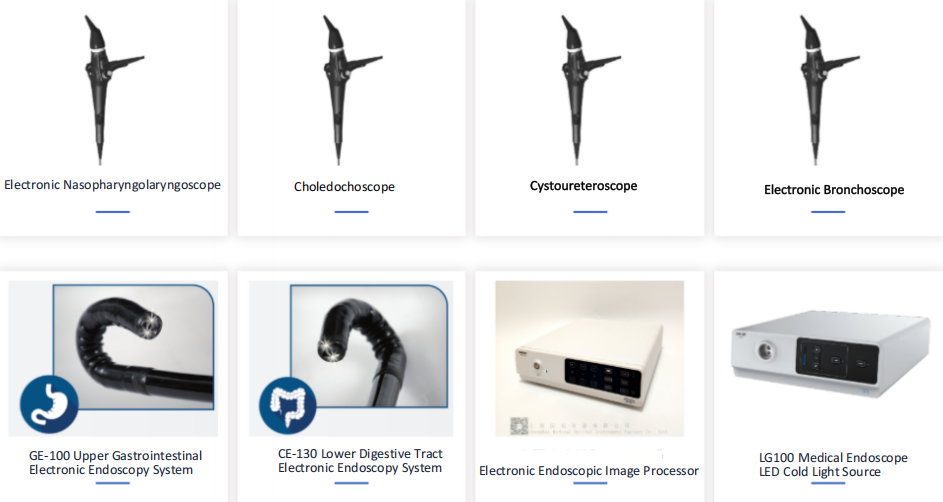
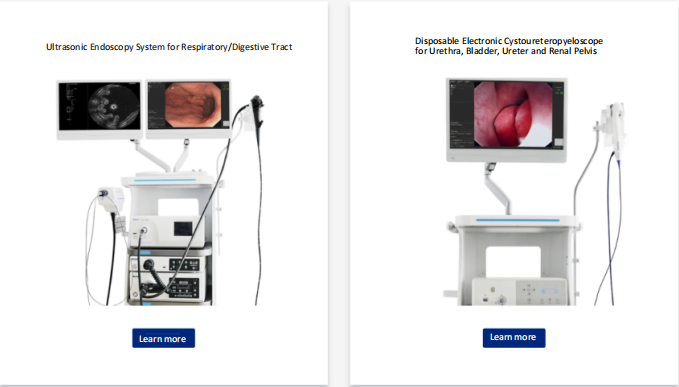
Hangzhou HANLIGHT Medical Technology Co., Ltd. is a pioneer and leader in medical endoscopy, having developed a series of innovative video endoscopes. HANLIGHT products include reusable electronic ureteroscopes, electronic cystoscopes, electronic nasopharyngolaryngoscopes, electronic cystoureteroscopes, electronic bronchoscopes, electronic choledochoscopes, and electronic portable intubation scopes. These products are widely used in urology, anesthesiology, ICU, ENT, respiratory medicine, and emergency departments.
Shanghai Oujiahua Medical Instrument Co., Ltd. has been a manufacturer and supplier of flexible endoscopes since 1998. We produce medical fiberoptic endoscopes, medical electronic endoscopes, industrial fiberoptic endoscopes, and industrial electronic endoscopes. The company actively absorbs top-tier endoscope technologies from both domestic and international sources, employing new materials and production techniques, resulting in significant improvements in product quality. Our mission is to manufacture high-quality products and provide comprehensive and complete after-sales service. “Reputation First, Quality First, and Customer First” is our solemn commitment and a principle we will always uphold.
Beijing Lepu Medical Imaging Technology Co., Ltd. (referred to as “Lepu Medical Imaging”) is a comprehensive independent enterprise under Lepu (Beijing) Medical Device Co., Ltd., integrating research, technology development, production, sales, and trade. Since its establishment in 2013, it has persisted in independent research and development while participating in extensive collaborations, achieving breakthroughs in the field of endoscopic diagnosis and treatment, mastering core intellectual property rights, and launching comprehensive endoscopic diagnosis and treatment solutions to serve China’s medical and healthcare industry.
Innovex Medical Group is a renowned healthcare group focusing on providing comprehensive solutions in the field of minimally invasive medicine, with innovation as its core value. INNOVES products and technologies are widely used in the diagnosis and treatment of diseases in urology, gastroenterology, respiratory medicine, gynecology, and general surgery. The INNOVES Medical Group consists of three independently operating enterprises specializing in minimally invasive consumables, disposable endoscopes, and energy equipment and consumables.
Hunan Reborn Medical Technology Development Co., Ltd. is a high-tech enterprise specializing in the research, development, production, and sales of medical devices, committed to promoting and selling internationally advanced medical products and technologies. Established in December 2006, the company is located in Zhuzhou High-tech Zone. The company regards product quality and innovation as its lifeblood. The current factory site covers an area of nearly 83,000 square meters, with a 100,000-class clean workshop, warehouse, and standard laboratory built according to YY0033-2000 standards. The purification area covers 22,000 square meters, including a laboratory area of approximately 1,200 square meters, equipped with a 10,000-class sterile laboratory, positive laboratory, and microbial limit laboratory. The company is a national “Specialized, Refined, Peculiar, and New Key Little Giant” enterprise, a “National High-tech Enterprise”, a “Provincial and Municipal Enterprise Technology Research Center”, a “Provincial Enterprise Technology Center”, an “Excellent Enterprise in the Medical Device Industry”, a “Hunan Little Giant” enterprise, a pilot enterprise for brand capability improvement of “Huxiang High-quality” small and medium-sized enterprises, a “Hunan Engineering Technology Research Center”, a “Hunan Well-known Trademark Brand”, and one of the key enterprises supported by the Hunan Provincial Government’s “13th and 14th Five-Year Plan” medical device planning. It is also a “Zhuzhou Small and Medium-sized Enterprise Brand Capability Benchmark Enterprise” and a “Zhuzhou Gazelle Enterprise”. The company currently has over 280 employees, including 60 R&D personnel.
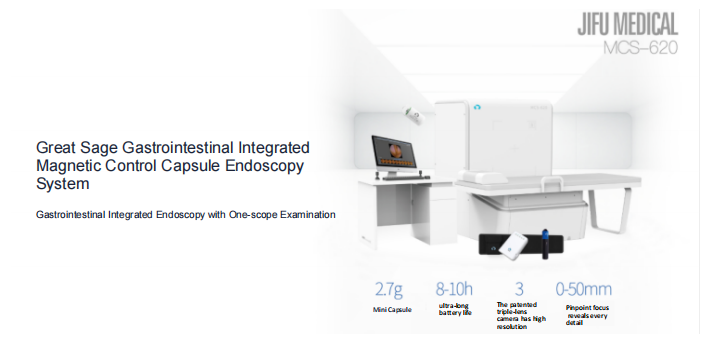
Founded in 2011, Shenzhen Jifu Medical Technology Co., Ltd. is a national high-tech enterprise specializing in the research, development, production, sales, and service of high-end gastrointestinal medical products.
The company’s headquarters is located in the High-Tech Industrial Park in Nanshan District, Shenzhen, and has established a modern production base in Guangming, Shenzhen. The company has established a medical device quality management system, passed Good Manufacturing Practice (GMP) inspection, and obtained ISO13485 quality management system certification.
The company has built a professional R&D team and an international R&D management platform, successively undertaking multiple technological innovation projects at the national and Shenzhen levels, and has obtained over 100 national patents. Adhering to independent innovation and the spirit of craftsmanship, after ten years of independent research and development, the company’s “Great Sage” magnetic-controlled capsule endoscopy system series products have obtained Class III Medical Device Registration from the National Medical Products Administration (NMPA), EU CE certification, and have received unanimous praise from medical institutions.
Established in 2009, Ankon Technologies is a high-tech enterprise specializing in the research, development, production, and operation of innovative medical devices in the field of gastrointestinal health. The company focuses on internationally leading medical technology innovation and is the pioneer and leader in magnetic-controlled capsule gastroscopy technology. We are committed to promoting comfortable and precise early screening of gastrointestinal diseases, developing intelligent gastrointestinal health management platforms, and assisting Healthy China initiative through a comprehensive digestive disease prevention, screening, diagnosis, treatment, and rehabilitation cycle.
Ankon’s gastrointestinal disease screening products (Ankon’s “Magnetic-controlled Capsule Gastroscopy System”) and constipation treatment products (VibraBot™ “Gastrointestinal Vibration Capsule System”) have filled gaps in global medical technology. Among them, the “Magnetic-controlled Capsule Gastroscopy System” has realized comfortable and precise gastric examination without endoscopy, obtaining Class III Medical Device Registration Certificate from the National Medical Products Administration and EU CE certification, and passing the US FDA De Novo Innovative Medical Device Registration. Currently, this product has been clinically applied in nearly 1,000 medical institutions across 31 provinces, municipalities, and autonomous regions in China, and has been exported to overseas markets.
Huiview Medical’s original aspiration is to develop an accessible, acceptable, non-invasive, painless, efficient, and accurate method for early diagnosis of esophageal diseases and early screening of esophageal cancer. Huiview Medical is committed to becoming a provider of comprehensive solutions for early screening, diagnosis, and treatment of gastrointestinal tumors, empowering primary hospitals to help patients receive high-quality and cost-effective early diagnosis and treatment of gastrointestinal tumors.
We, Jiangxi Zhuoruihua Medical Instrument Co.,Ltd., is a manufacturer in China specializing in the endoscopic consumables, include GI line such as biopsy forceps, hemoclip, polyp snare, sclerotherapy needle, spray catheter, cytology brushes, guidewire, stone retrieval basket, nasal biliary drainage cathete etc. which are widely used in EMR, ESD, ERCP, compatible with the all the gastroscopy, colonoscopy and bronchoscopy in the market. And Urology Line, such as ureteral access sheath and ureteral access sheath with suction, disposable Urinary Stone Retrieval Basket, and urology guidewire etc, compatible with the all the ureteroscopy in the market.
Our products are CE certified and with 510K approval, and our plants are ISO certified. Our goods have been exported to Europe, North America, Middle East and part of Asia, and widely obtains the customer of the recognition and praise!
Post time: Oct-10-2025